Exploring Nanotechnology: Fold, Roll, & Stack Your Way to Super-Strong Materials
Abstract
Advertisements for high-tech sports gear or the latest and greatest outdoor material promise lighter and stronger products every season. Is it a scam? How can engineers keep creating materials that are both lighter and stronger than anything known so far? The answer is in the nanoscale! Using nanotechnology, scientists can play around with the detailed structure of matter, leading to a whole new range of materials, some with amazing qualities. In this science project, you will get a glimpse into the exciting nanoworld by exploring how arranging material in different ways can drastically change the strength of the final product. Get ready to learn some basic origami, and if you already know some, you'll be surprised how those skills will come in handy!Summary
Sabine De Brabandere, PhD, Science Buddies
Objective
Study how different arrangements of paper (representing carbon nanostructures) result in materials with different strengths by folding, rolling, and stacking origami paper.Introduction
If you've ever been camping or have a fireplace at home, you've probably noticed that after the logs have burned, all that's left is a pile of ashes. The ashes contain a lot of soot, shown in Figure 1, below. So what does this black, flaky substance have to do with nanotechnology and stronger materials? You're about to find out, and be prepared for some surprises along the way!
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
|
| Figure 1. Soot, a material found in the leftovers of burned wood. |
First, let's learn about the nanoscale. The nanoscale is the scale at which the basic building blocks (which can be molecules or atoms ) of ordinary material around us reveal themselves. In other words, it is the scale where we can observe objects that have dimensions of a couple of nanometers. So, how small is one nanometer? Any idea? In mathematical terms, a nanometer (abbreviated nm) is 1 billionth of a meter. Picture this: if you divided 1 meter into 1 billion equally long pieces, one of those sections would be 1 nm. Talk about small! The following facts might give you a better idea:
- A typical virus is about 30–50 nm long.
- A human hair measures roughly 50,000–100,000 nm in diameter.
- A sheet of paper is about 100,000 nm thick.
- Fingernails grow about 1 nm every second.
Getting back to the soot, at the nanoscale, you will mainly find one specific building block called carbon, arranged in different ways. Most of the carbon building blocks found in soot are arranged in sheets that can shift easily with respect to one another, giving it its easily breakable, flaky character. Imagine holding a stack of small papers between your thumb and index finger; it would be very easy to move them around, causing them to appear flaky. Carbon has been used in this form for decades as a lubricant (a substance that minimizes friction), as a pigment in paint, and as the graphite center of pencils. Figure 2, below, will help you visualize this.
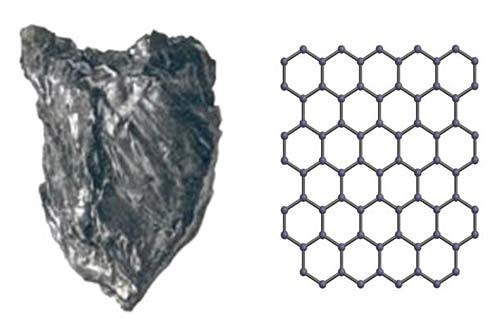 Image Credit: wpclipart.com / Public domain Image Credit: wpclipart.com / Public domain
|
| Figure 2. Graphite (left) is made of carbon that is arranged in sheets, which can shift easily with respect to one another. A visualization of how the carbon building blocks (represented by tiny black spheres) are arranged to form a sheet is shown on the right. The connections between the spheres represent the bonds that hold them together. |
Recently, scientists found that soot also contains a small amount of carbon arranged in hollow spheres and tubes. These are called fullerenes. Put to the test, fullerenes are many times stronger than steel. They are lightweight, and some forms conduct electricity better than the traditional copper wire. See where it gets interesting? Since fullerenes are strong and lightweight, they can be used to make super-strong, lightweight tennis rackets or bike frames.
The two most well-known forms of fullerenes are the nanotube and the buckyball. Nanotubes are like a single layer of graphite rolled in a cylindrical tube.
The buckyball or Buckminsterfullerene looks more or less like a soccer ball. Figure 3, below, shows both. Of course, all these visualizations are not true to size; a buckyball is only a few nanometers in diameter! Blow it up 30,000 times and it will still only be the width of a typical human hair.
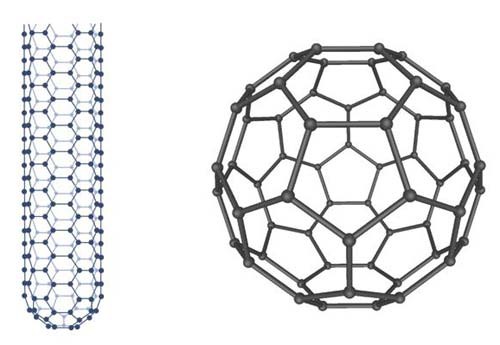 Image Credit: Wikimedia Commons / Public domain Image Credit: Wikimedia Commons / Public domain
|
| Figure 3. Visualization of how the carbon building blocks can be arranged to form hollow structures called fullerenes. Here, a nanotube (left) and a buckyball (right) are represented. The tiny black spheres represent the carbon building blocks, while the lines between the spheres represent the bonds that hold them together. |
You may be surprised at a very different form in which carbon can present itself: as a diamond! Put under extreme pressure, the carbon building blocks found in soot will spontaneously pack together closely to form the translucent, hard material we call diamond. This is the densest form of carbon, packing the most basic building blocks of carbon in the smallest area. Look at Figure 4, below, and imagine how the soot shown in Figure 1 transforms into this beautiful, hard material.
 Image Credit: wpclipart.com / Public domain Image Credit: wpclipart.com / Public domain
|
| Figure 4. The basic building blocks of carbon put under extreme pressure will arrange themselves into a material we call diamond, depicted in the left figure. The right figure shows how the carbon building blocks (represented by tiny black spheres) are arranged to form diamond. The lines between the spheres represent the bonds that hold the carbon together. |
Table 1, below, sums up what you have learned so far.
| Material Name | Carbon Arrangement | Characteristics | Applications |
| Graphite | Stacked sheets | Breakable and flaky | Pencils Lubricant Ink |
| Fullerenes | Hollow structures | Lightweight and strong | Bicycle frames Tennis rackets |
| Diamond | Densely packed | Heavy and strong | Jewelry Diamond saw blades |
The fullerenes, together with their amazing characteristics, are discovered using nanotechnology. Scientists are currently able to manipulate and put together carbon in the laboratory, allowing them to create different fullerenes. If we can get so many different uses out of different arrangements of carbon, imagine how much more we can do with controlled arrangements of all the existing building blocks. Of course, that currently requires very expensive scientific equipment. But, what if you arrange everyday materials in different ways; for example folding, rolling, and stacking sheets of origami paper? Do you think the resulting structures will have different characteristics, like strength?
A few important notes before you start. You will be making different models of carbon structures using origami paper. Models are objects that scientists construct to help them understand things in nature. For example, the origami paper structures in this science project are models representing carbon nanostructures. They will help you understand what happens in the nanoworld. Paper models that you can hold in your hands and see with your naked eye are much more accessible and real to us than actual nanostructures. However, these paper models do not behave exactly like the actual nanoscale structures. The physics of forces (how things push and pull on each other) changes dramatically at different scales (the nanoscale, or nanometers, vs. our everyday scale, or meters). Think of a solar system model. The solar system is much, much bigger than our everyday scale (roughly gigameters, or 1 million meters). Do planets in a solar system model move like the real planets do? Do they revolve around the model sun like real planets revolve around the sun? Do they "float" in space like real planets do? Could we conclude that as our model planets do not float in space, the real ones do not either? Of course not. So be aware that the conclusions you draw from your models might be different from conclusions drawn by scientists studying actual carbon structures. That said, get ready to fold, roll, and stack!
In this science project, you will create three different constructions of six origami paper sheets each and compare their relative strengths. The number of pennies the structure can comfortably carry in a cup hanging from the structure before showing "cracks" (further explained in the Procedure tab) will be used as the measure of strength.
Terms and Concepts
- Soot
- Nanoscale
- Nanometer
- Carbon
- Fullerene
- Nanotube
- Buckyball or Buckminsterfullerene
- Nanotechnology
- Model
- Forces
Questions
- Can you find more applications of the different types of materials made from carbon and only carbon?
- Can you rank a volume of 1 cm³ of graphite, 1 cm³ of material made of pure fullerenes, and 1 cm³ of diamond from lightest to heaviest? Hint: Carbon is densely packed in diamond and is arranged in hollow structures to form fullerenes.
- In nanotechnology, the term bottom-up method is where you start with nanoscale particles (atoms or molecules) to create new or to enhance existing materials. Is the study of fullerenes to enhance materials using the bottom-up method?
- Can you find some products readily available today that are enhanced by fullerenes?
- In addition to the ones discussed in the Introduction, which industries or products benefit from ever stronger, lighter materials? Can you think of any science-fiction projects or ideas that might become realistic with the invention of even stronger, lighter materials?
Bibliography
- Woodford, Chris. (2007). Nanotechnology. Retrieved May 14, 2013.
- NASA. (2011, January 28). Nanotechnology at NASA. Retrieved May 13, 2013.
- National Nanotechnology Coordination Office. (n.d.). What It Is and How It Works. Retrieved March 4, 2021.
Check out the following link for instructions on how to fold an origami cube:
- Origami-instructions.com. (n.d.). Origami cube. Retrieved May 13, 2013.
The following website explains how to calculate the average:
- MathIsFun.com. (2012). Definition of: Mean. Retrieved July 23, 2013.
Materials and Equipment
- Cup, plastic disposable
- 1-hole puncher
- Curling ribbon (24 inches long), available in the party supply section of a home goods or grocery store
- For an image of the ribbon type, see Figure 5 in the Procedure tab
- Ruler
- Scissors
- Origami paper, 7- by 7-inch square or larger (60 sheets) available in craft stores
- Tape
- Chair or table with right-angle edges (2 identical)
- Pennies (250)
- Optional: Kitchen scale
- Lab notebook
Experimental Procedure
Preparing Your Materials
- First, make the cup to hold the pennies, as follows:
- Make two holes with the 1-hole puncher on opposite sides of the plastic cup, near the top edge.
- Measure and cut about 24 inches of the curling ribbon. Tie each end of the ribbon to one hole of the cup. Tip: Do not cut the excess ribbon too short; it is needed to ensure the knots will stay tight.
- The result should look like the cup shown in Figure 5, below.
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
|
| Figure 5. This cup with curling ribbon attached will be used to measure the strength of structures by hanging it over the structure and measuring the amount of weight it can hold. |
- Create one cube from six origami papers.
- Choose six origami papers and fold them into one cube. If you have never folded an origami cube, the instructions provided by Origami-instructions.com will help you transform your six flat sheets of paper into a single cube.
- Create six rolls from six origami papers.
- Roll one origami paper at a time. Make the rolls tight enough that the tube has two layers of paper all the way around. If you are using origami squares of 7 by 7 inches, the diameter of your roll will be 1 1/8 inch.
- Adjust your roll so the edges of the paper line up, as shown in Figure 6.
- Secure the roll with a small piece of tape.
- Repeat steps 3.a.–3.c. for the other five origami papers.
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
|
| Figure 6. A piece of origami paper rolled tightly enough to be two layers thick, and secured with a small piece of tape. |
- Stack six origami papers on top of each other.
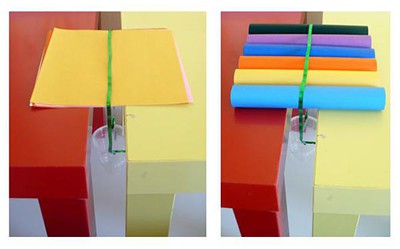
- Figure 7, below, shows the three different structures, each made of six origami sheets. Predict which one of these will be the strongest and record your prediction in your lab notebook.
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
|
| Figure 7. A single origami cube folded from six origami papers (left), six rolls formed from six individual origami papers (middle), and a stack of six origami papers (right) are the three different structures that will be tested for their strength in this nanotechnology science project. |
Taking Your Measurements
- Create a data table, similar to Table 2 below, in your lab notebook. It will be used to record your findings. Note to leave enough open lines as you do not know yet how many pennies the strongest structure will carry.
| Trial 1 | Stacked Sheets | Rolls | Cube |
| Can hold (Yes/No) | |||
| 0 pennies | |||
| 5 pennies | |||
| 10 pennies | |||
| 15 pennies | |||
| ... | |||
| Optional: Maximum weight carried (in grams [g]) | |||
- Place the two identical chairs or tables parallel to each other, leaving a gap of about 1 1/2 inches between them. Make sure your cube can comfortably bridge the gap between the chairs or tables.
- Start with the stacked sheets structure.
- Place the structure over the gap with the cup hanging from it, down into the gap. Figure 8, below, can help you visualize the setup. Aim to have an equal amount of structure resting on each chair or table.
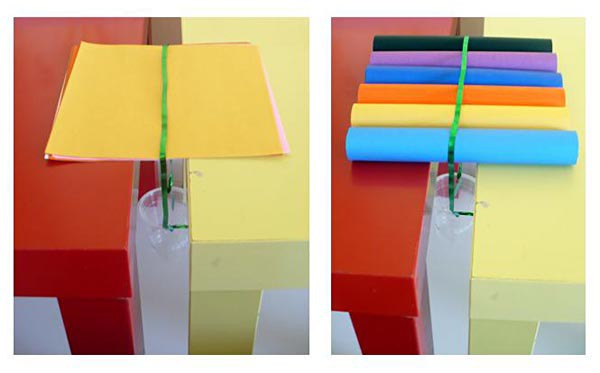 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
|
| Figure 8. Figures demonstrating the setup used to measure the strength of two of the structures. The stacked sheets structure (left) and the rolls structure (right) bridge a gap of 1 ½ inches between two identical chairs or tables. A cup that will carry the weight is hanging from the structure. |
- Test if the structure can hold the empty cup. Record your findings in your data table. Move to step 13 if your structure cannot hold the empty cup. For this science project, seeing a crack or fold somewhere in the structure indicates the structure cannot carry the weight.
- Add weight until the structure cracks, as follows.
- Hold the cup so the weight of it is in your hands, but so the structure remains in place. Place five pennies in the cup and gently bring the cup back down and let go of it.
- Note if the structure can hold the extra weight. Pay attention to the details. Do you see your structure bend? If so, does it return to its original form if you lift the cup with pennies? For this science project, seeing a crack or fold somewhere in the structure indicates the structure cannot carry the weight. Figure 9, below, shows the rolls and the cube both carrying a weight. Note that the rolls in the picture show signs of carrying weight—they bunch together—but are not showing any cracks yet. Record your findings in your data table.
- Repeat steps 11.a. and 11.b. until your structure shows a crack under the weight.
- If you have a kitchen scale available, weigh how much the structure could carry before showing any cracks:
- Remove the cup with pennies from the structure, then take out five pennies (since the structure could not hold the weight with the last five pennies added, but it could before you added them).
- Measure the weight of the cup with pennies.
- Record the measurement in the data table in the line "Maximum weight carried (in grams [g]).
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
|
| Figure 9. Figures showing two structures (rolls [left] and cube [right]), each carrying a weight. Note that the rolls get deformed carrying the weight, but do not show any signs of cracks or folds carrying this weight. |
- Remove the structure and empty the cup.
- Repeat steps 9–12 using the six rolls as the structure, placed parallel to each other, as shown in Figures 8 and 9, above.
- Repeat steps 9–12 using the cube as the structure.
- This completes one trial. Repeat steps 2–14 two more times for a total of three trials (three for each structure). Before you start trial 2, you will have to remake the structures. After a structure has cracked once, it will be permanently weakened, so you cannot use it again for future tests. You will need to make new structures for each new trial.
Analyzing the Measurements
- Create a summary table similar to Table 3, below, in your lab notebook.
| Stacked Sheets | Rolls | Cube | |
| Number of pennies or weight [g] | |||
| Trial 1 | |||
| Trial 2 | |||
| Trial 3 | |||
| Average | |||
- Fill in your summary table by retrieving the maximum number of pennies or weight (g) the structures could hold in each trial from your data tables that were similar to Table 2.
- Calculate the average number of pennies or average weight each structure could hold over the three trials. If you need a refresher on how to calculate an average, check out the reference in the Bibliography.
- Does any of the data stand out? Was one structure stronger in all three trials? Were the variations between trials small or big? Are the findings in alignment with what you expected?
- Are your findings similar to findings of carbon structures in the nanoworld, where carbon sheets are found to be less strong than the hollow fullerenes?
Ask an Expert
Global Connections
The United Nations Sustainable Development Goals (UNSDGs) are a blueprint to achieve a better and more sustainable future for all.
Variations
- Include different structure sizes; for example, you could test a small, medium, and large cube. How does size affect the strength of the structures? Create a graph of strength vs. size.
- Include other paper structures, like an origami icosahedron, in your comparison.
- The density or weight per volume plays an important role when engineering new materials. For example, even though steel is sometimes stronger than specific fullerenes, it is much denser and thus heavier, making it less useful for applications like tennis rackets (people prefer a lightweight racket). Can you factor density (weight per unit volume) of your materials into your analysis? Which one of your structures would have the least/highest density? How would this relate to nanotechnology, where scientists like to invent lightweight, strong materials?
- Test what happens if you change the gap distance between the edges of your tables or chairs. When you move the tables closer together, you are effectively making the structures shorter (the part that is just resting on the table won't bend, but the part suspended over the air might). This is a way to test the effects of size without making new structures. Does the gap width affect the amount of weight your structures can hold? Create a graph of weight vs. gap distance.
- What happens if you use tape to tape together the edges of your paper sheets or tubes? Engineers call this a boundary condition. The same structure can have a different strength if its boundary conditions change. For example, if you tape the edges of your flat sheets together, they might not be able to slide around as easily. In the nanoworld, boundary conditions relate to how the different carbon structures bond together.
Careers
If you like this project, you might enjoy exploring these related careers: