Burning Calories—Literally!
Summary

Overview
Your students might know that they can burn calories, but do they know what a calorie really is? In this fun lesson plan, your students will measure the energy content of food by literally burning it using a device called a calorimeter that they will design and build themselves. This will get your students thinking about the chemistry of energy transfer as well as good nutrition, and gives a whole new meaning to the phrase "burning calories!"Learning Objectives
- Understand the Calorie as a unit of food energy.
- Explain the processes that allow a calorimeter to measure the energy stored in food.
- Use a homemade calorimeter to measure and calculate the energy content of a food item.
- Explain the relationship between calories, energy, and good nutrition.
NGSS Alignment
This lesson helps students prepare for these Next Generation Science Standards Performance Expectations:- MS-PS3-3. Apply scientific principles to design, construct, and test a device that either minimizes or maximizes thermal energy transfer.
- MS-PS3-4. Plan an investigation to determine the relationships among the energy transferred, the type of matter, the mass, and the change in the average kinetic energy of the particles as measured by the temperature of the sample.
|
Science & Engineering Practices
Constructing Explanations and Designing Solutions.
Apply scientific ideas or principles to design, construct, and test a design of an object, tool, process or system.
Engage in Argumentation from Evidence. Construct, use, and present oral and written arguments supported by empirical evidence and scientific reasoning to support or refute an explanation or a model for a phenomenon. Mathematical and Computational Thinking. Apply mathematical concepts and/or processes (e.g. ratio, rate, percent, basic operations, simple algebra) to scientific and engineering questions and problems. Connections to Nature of Science Scientific Knowledge is Based on Empirical Evidence. Science knowledge is based upon logical and conceptual connections between evidence and explanations. |
Disciplinary Core Ideas
PS3.A: Definitions of Energy.
Temperature is a measure of the average kinetic energy of particles of matter. The relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter present.
PS3.B: Conservation of Energy and Energy Transfer. The amount of energy transfer needed to change the temperature of a matter sample by a given amount depends on the nature of the matter, the size of the sample, and the environment. Energy is spontaneously transferred out of hotter regions or objects and into colder ones. |
Crosscutting Concepts
Energy and Matter.
Energy may take different forms (e.g. energy in fields, thermal energy, energy of motion).
The transfer of energy can be tracked as energy flows through a designed or natural system. Systems and System Models. Models can be used to represent systems and their interactions—such as inputs, processes, and outputs—and energy and matter flows within systems. |
Materials
For each group of 2–4 students, you will need:
- Calorimeter Kit, available from our partner
Home Science Tools. The kit includes:
- Large tin can, 6 3/16 inches by 7 inches; no lid, no bottom
- Small tin can, 3 3/8 inches by 4 1/16 inches; no lid
- Wooden dowel, 12 inches by 1/4 inch
- Bottle cork
- Aluminum pie pan, 8 inch diameter
- Sewing needles, sharps size 10 (4)
- 20 gauge craft wire, 10 inch length (2)
- Plastic graduated cylinder, 250 mL
- Immersion thermometer
- Safety glasses
- Additional safety glasses, so each student has one pair
- Water
- Long matches, a Bunsen burner, or a multipurpose lighter
- Digital scale with 0.1 g increments, like this scale available from Home Science Tools
- Oven mitts, at least 1 per group.
- Pieces of a food to test (4–5) Include one that is rich in healthy fats like nuts, and one that is lower in fat but rich in refined sugar like marshmallows. Dry items with a relatively high oil content and those that trap air will generally work better. Examples are:
- Nuts (cashews, peanuts, etc.)
- Popcorn
- Marshmallows
- Croutons
- Dry pet food
- Croissant
- Cheerios®
- Cloth or paper towels
- Calculator
- Optional: material to prop up the can and leave an opening for air flow at the bottom
- Optional: Aluminum foil
To prepare ahead, you will need:
- Transparent containers, 1 per food item that will be tested. Containers will need to hold 100 g of food.
Disclaimer: Science Buddies participates in affiliate programs with Home Science Tools, Amazon.com, Carolina Biological, and Jameco Electronics. Proceeds from the affiliate programs help support Science Buddies, a 501(c)(3) public charity, and keep our resources free for everyone. Our top priority is student learning. If you have any comments (positive or negative) related to purchases you've made for science projects from recommendations on our site, please let us know. Write to us at scibuddy@sciencebuddies.org.
Background Information for Teachers
This section contains a quick review for teachers of the science and concepts covered in this lesson.With increased public emphasis on lifelong good nutrition habits to combat obesity and related diseases, it can be helpful for students to demystify the nutrition facts label (Figure 1) and the energy or Calorie content of food.
 Image Credit: FDA / Public Domain
Image Credit: FDA / Public Domain
Figure 1. Nutrition facts label.
Our bodies need energy to survive and thrive. What we eat is broken down to release its stored energy, which we use to survive, grow and be active; whatever is leftover is stored as fat. Our food comes in many forms, but it basically consists of three main types of nutrients: sugars (also called carbohydrates), proteins, and fats (also called lipids). These complex food molecules store energy in the chemical bonds that hold them together. This type of energy is called chemical energy and can be expressed in Calories (note the capital "C" used to indicate one kilocalorie, or one thousand calories, with a lowercase "c"), the unit of energy used in the United States to quantify food energy. Inside our bodies, ingested food molecules undergo a series of changes—oxidations, which is a type of burning—that break the bonds and slowly release stored energy. In this lesson, you and your students will break down food much more rapidly by burning it in air. You will use a calorimeter to catch and measure the released energy.
A calorimeter, as shown in Figure 2, is an instrument used to measure the amount of heat created by a chemical or physical change. Heat is the type of energy that flows between two bodies due to a difference in temperature. Students will design and build their own calorimeters to measure the heat created by releasing the energy stored in food items. The basic idea is to release all the stored food energy at once and capture all the released heat with a reservoir of water. Measuring the change in temperature of the water will allow you to calculate the energy required to heat up the water. As this energy comes from breaking down food, the calculation will reveal the actual amount of energy provided, or the energy originally stored in the food. However, this is only true if all the energy released by the food is used to heat the water, and none of it gets "lost."
 Image Credit: Wikimedia commons user Akshat Goel / Creative Commons Attribution Share-Alike 3.0
Image Credit: Wikimedia commons user Akshat Goel / Creative Commons Attribution Share-Alike 3.0
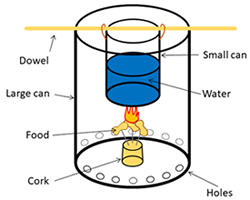 Image Credit: Sabine De Brabandere, Ben Finio, Science Buddies / Science Buddies
Image Credit: Sabine De Brabandere, Ben Finio, Science Buddies / Science BuddiesA diagram shows a homemade calorimeter that burns food at the bottom of a can which heats up a smaller can of water above. A small can of water is suspended from a dowel that lays across the opening of a larger can. A cork at the bottom of the large can is used to hold burning food underneath the can of water. Holes are drilled along the bottom edge of the larger can to allow air to enter.
Figure 2. Setup of a calorimeter used by scientists (Picture created by Akshat Goel, Wikimedia commons), and a schematic view of a calorimeter students could build.
Calorimeters that are used by scientists are made so that practically all of the energy released during the chemical or physical change is captured by the water. The homemade version will not reach the same efficiency in catching the heat released when burning the food; in other words, only a fraction of energy stored in the food will be converted to thermal energy of the water and measured by your calorimeter. For example, some of the energy might go into heating up the surrounding air instead of the water. Capturing even half of the energy released (an efficiency of 0.5 or 50%) is acceptable for a homemade calorimeter, as it is very difficult to transfer all the energy that is released to the water reservoir. Even with low efficiencies, this lesson allows your students to rank different foods based on their Calorie content. With reasonable accuracy, it allows your students to calculate the ratio of Calorie content of different foods.
With the homemade calorimeter, your students will measure the temperature change (ΔT) of a reservoir of water due to the burning of a food item. Multiply this by the mass of the water (mwater) that has been heated, and the specific heat capacity of water c (0.001 Cal/(g °C)) to calculate the amount of energy used to heat up the water (Qwater). This is also shown in Equation 1.
Equation 1:
The following example where we burn 1.1 g of almonds will make the equation clear. We start out with 150 milliliters (mL) of water in the calorimeter. Since 1 mL of water has a mass of exactly 1 g, this water has a mass of 150 g (mwater = 150 g). If initially the temperature of the water is 20.0°C, and after burning the nuts in the calorimeter we measure a water temperature of 33.3°C, then the heat captured by the calorimeter is:
If all the energy released is used to heat up the water, this would be a measure of the energy stored in the nuts we burned. In reality, some energy will get lost so the real energy content of the burned food is likely to be higher.
To compare different foods, we need to calculate the energy released by a standard amount of food. We do this by dividing the calculated energy by the mass difference of the food item before and after burning. This specific energy, or the energy content per unit of mass (QFood, 1 g), is what allows us to identify Calorie-rich food items. In the above example, (QAlmond, 1 g) equals 2.0 Cal/1.1 g, or 1.81 Cal/g.
Food as a source of energy contains two aspects: a quantitative aspect focusing on the amount of energy in food and how this might vary depending on the type of food and, a qualitative aspect focusing on whether some sources of food energy are healthier than others. This lesson emphasizes the quantitative aspect of food. The Variations section includes information on how you can explore the health and nutritional aspect of food.
Eating a balanced, nourishing diet and getting enough exercise are fundamental to good health. The energy balance between what we take in and what we use is important for health, but it is not the only component of a healthy diet. To adequately nourish our bodies, our diets need to provide items from all the food groups in sufficient amounts. If one needs to cut down on Calories, it is a good idea to start cutting down on processed foods and refined sugars, as these have little nutritional value while still adding to the energy balance.





