Green Chemistry Lesson Plans (7 results)
The goal of green chemistry is to make sure that chemical processes and products are produced in ways that reduce or eliminate the use and creation of toxic material. It encourages the use of as little energy and as few materials as possible and emphasizes using renewable resources. The idea is to be environmentally friendly and sustainable from the beginning of the process rather than figure out how to treat waste after a product is made or discarded.

Green chemistry can be applied to improve existing products or processes. For example, green chemistry is being applied to clothing manufacturing to make the creation of synthetic fabrics more environmentally friendly and ensure that the dyes used to color all clothes use fewer water resources and do not pollute. The same green chemistry principles can be used to make new products like biodiesel made from plants, to replace diesel made from fossil fuels.
The twelve principles of green chemistry provide a framework for scientists and engineers to follow when designing new products or improving existing materials and processes.
- Waste prevention: Instead of treating or cleaning up waste in the end, prevent it from being made.
- Atom economy: Incorporate as much of the starting materials (atoms) into the final product as possible.
- Less hazardous chemical synthesis: When creating complex compounds from simpler materials (chemical synthesis), focus on using and creating materials that do not harm humans or the environment.
- Design safer chemicals: When designing chemical products, make ones that are effective (do the job intended) but have little or no toxicity.
- Safer solvents and auxiliaries: The use of chemicals like solvents, separation agents, etc., that help a chemical process should be either eliminated or, if that isn't possible, made as environmentally friendly as possible.
- Design for energy efficiency: Run chemical reactions at room temperature and pressure whenever possible as it takes extra energy to heat, cool, or alter pressure.
- Use renewable feedstocks: As much as possible, raw materials (also called feedstocks) should come from renewable sources instead of ones that can be depleted. Examples of renewable feedstocks include agricultural products and waste from other processes.
- Reduce derivatives: Derivatization, like using blocking or protecting groups or any temporary modification to a compound, should be avoided if possible. Creating derivatives uses additional reagents and may create more waste.
- Use catalysts: Minimize waste by using catalytic reactions. Catalysts are effective in small amounts and can carry out a single reaction many times. They are preferable to stoichiometric reagents, which are used in excess and carry out a reaction only once.
- Design for degradation: Design chemical products to break down to harmless substances after use so that they do not accumulate in the environment.
- Real-time pollution prevention: Include in-process, real-time monitoring and control during synthesis to minimize or eliminate the formation of byproducts (unwanted products).
- Minimize the potential for accidents: Choose chemicals and their physical forms (solid, liquid, or gas) to minimize the potential for chemical accidents, including releases to the environment, explosions, and fires.
|
Select a resource
Sort by
|
Lesson Plan
Grade: 9th-12th
7 reviews
This lab discusses types of reactions and replaces traditional reaction experiments involving chemicals such as lead (II) nitrate, barium chloride, and silver nitrate with greener alternatives. This lab is designed to challenge students to identify types of chemical reactions and distinguish between those that use safer, less hazardous chemicals and those that are more dangerous. Students will make a choice as to which reaction they will perform using the 12 Principles of Green Chemistry. They…
Read more
Featured
Lesson Plan
Grade: 6th-8th
7 reviews
Junkbots are easy-to-build robots that you can make using a simple circuit and some recyclable materials. In this lesson, your students will learn about engineering design as they compete to build the fastest robot. No previous robotics experience is required!
Read more
NGSS Performance Expectations:
Lesson Plan
Grade: 6th-8th
1 review
These lessons use open-ended exploration to introduce students to biopolymers and the chemistry behind cross-linking. Students will draw inspiration from biology and use authentic scientific practices to design and create colorful string creations from a natural polymer, alginate.
Learning Objectives
Students will:
Engage in authentic science practices through open-ended exploration.
Create a string using polymers harvested from living algae.
Manipulate the properties of a…
Read more
New
Lesson Plan
Grade: 6th-12th
Create a two-part system for filtering greywater. Teams will focus on communication and systems engineering as they build separate components to filter solid and liquid waste and then combine them into one device.
Learning Objectives
Students will:
Consider the potential effects of drought and how greywater could be part of the solution.
Design a system for filtering out solid waste or liquid waste.
Consider effective communication strategies with their team.
Collaborate on their design…
Read more
Lesson Plan
Grade: 6th-12th
2 reviews
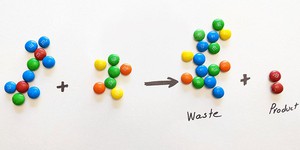
In this lesson, students will do a simple exercise with M&Ms to understand what environmental impact factor (E-factor) is, how it applies to chemical processes, and how waste from chemical reactions can be reduced by applying the principles of green chemistry.
Learning Objectives
Students will:
Understand strategies for reducing waste
Perform an exercise which has them practice E-factor
Relate the exercise to chemical processes
NGSS Alignment
MS-PS1-3.
Gather and make…
Read more
Lesson Plan
Grade: 6th-8th
Look around and you will see fabrics everywhere, from clothes to upholstery, in a wide range of colors. In this chemistry lab students will dye strips of fabrics to explore how variables like pH and fiber type influence fabric colors. Students will also look at the life cycles of natural and synthetic fibers and apply green chemistry principles to understand how science can help make advances towards a "greener" textile industry.
Learning Objectives
Students will:
Understand that synthetic…
Read more
Lesson Plan
Grade: 9th-12th
Educational Goal
To understand:
How polylactic acid (PLA) plastic is an example of green chemistry technology particularly pollution prevention and designing safer chemicals
Student Objectives
Students will:
Learn about renewable "corn" plastic is made from polylactic acid
Recycle the polylactic acid cup into a new product: a cleaning solution
Conduct a saponification reaction
Analyze PLA against the 12 principles of green chemistry
(Optional) Verify the contents of their…
Read more
Lesson Plan
Grade: 5th-12th
2 reviews
Where does CO₂ come from and how does excess carbon dioxide in the atmosphere affect the ocean and aquatic life? In this lesson students are introduced to the carbon cycle and explore pH and acidification with hands-on experiments. They then connect their experimental data with real-world data to evaluate claims about carbon dioxide and ocean acidification. Finally, students are introduced to how different companies and research groups are using green chemistry to build carbon capture…
Read more
Lesson Plan
Grade: 9th-12th
How does a solar cell work? In this green chemistry lesson plan, students will build and test their own dye-sensitized solar cells using dye from blackberries. Along the way, they will learn about the principles of green chemistry and evaluate how solar cell manufacturing can go green.
Read more
NGSS Performance Expectations:
|